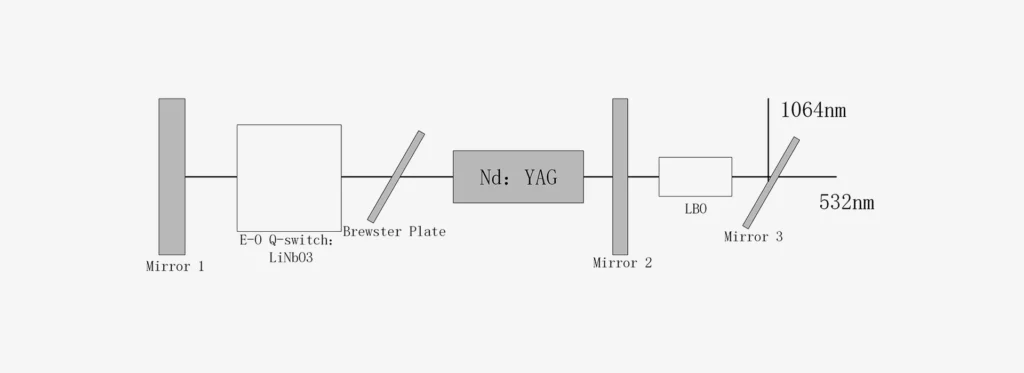
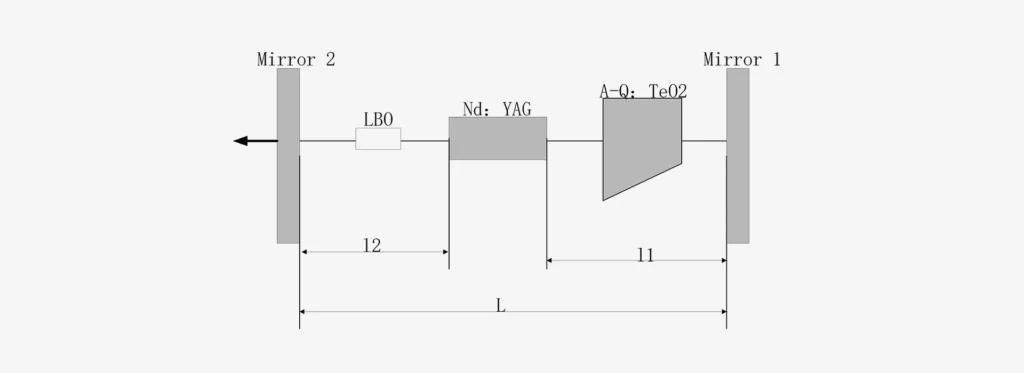
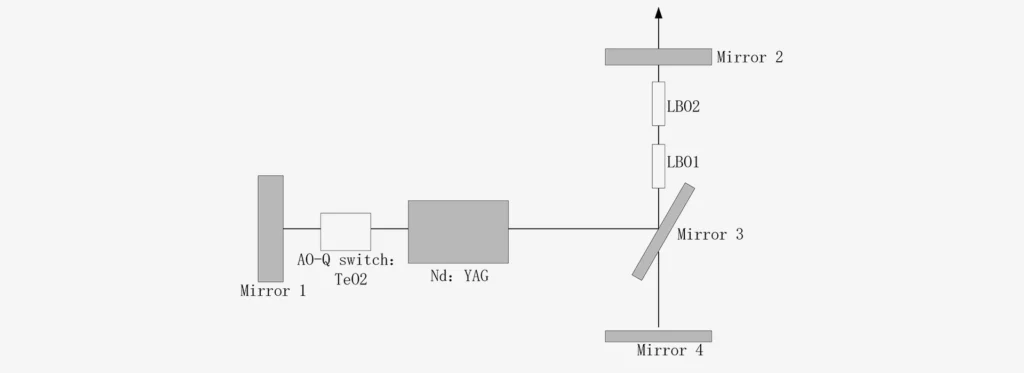
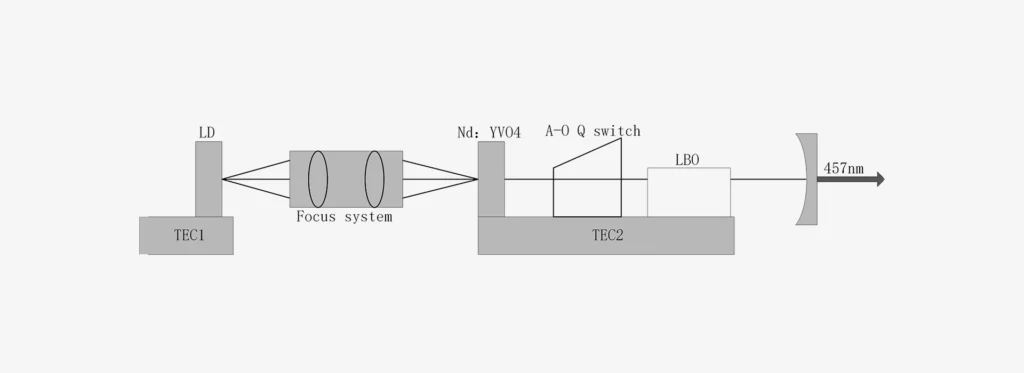
LBO (Nonlinear Crystal)

LiB3O5 (Lithium Triborate) nonlinear crystal is one of the most excellent nonlinear optical crystals found so far that can be used for non-critical phase matching laser frequency doubling.
It has good nonlinear optical and stable physical and chemical properties, which is especially important because its dispersion amount is sensitive to temperature changes.
Due to its large damage threshold, it can achieve non-critical phase matching during the frequency doubling process, which means it can achieve high-power fundamental pumping. Also, longer optical crystals can be used, which are undoubtedly useful for obtaining high-power frequency doubling lasers.
At 1.064 μm light, the effective SHG coefficient of the LBO nonlinear crystal is three times higher than that of the KDP.
The optical damage threshold of the LBO is the highest among the commonly used inorganic nonlinear optical crystals. Therefore, it is among the best choices for high-power second harmonic generators and other nonlinear optical applications.
Features of LBO Nonlinear Crystal:
- High optical uniformity
- Wide transparent area
- Wide tunable wavelength range
- low sensitivity to moisture
- Wide receiving angle, small discrete angle
- Spectral Noncritical Phase Matching (NCPM) close to 1300 nm
- Class I, II Non-Critical Phase Matching (NCPM) Wideband Range
- High damage threshold (1053 nm laser with a pulse width of 1.3ns can reach 10GW/cm2)
- High frequency doubling conversion efficiency (equivalent to 3 times that of KDP crystal)
Physical and Chemical Properties
| Attribute | Numerical |
| Chemical Formula | LiB3O5 |
| Crystal Structure | Rhombic, Space GroupPna21,Point Group mm2 |
| Lattice Constant | a=8.4473Å ,b=7.3788Å, c=5.1395Å, Z=2 |
| Mass Density | 2.47 g/cm3 |
| Mohs Hardness | 6 |
| Melting Point | About 834°C |
| Thermal Conductivity | 3.5W/m/K |
| Birefringence | Negative Biaxial Crystal:λ=0.5321μm,2Vz =109.2˚ |
Nonlinear Optical Properties
| Attribute | Numerical |
| SHG Phase Matching Range | 551 ~ 2600nm (Type I);790-2150nm (Type II) |
| NLO Coefficient | deff(I)=d32cosΦ(Type I in the XY Plane) |
| deff(I)=d31cos2θ+d32sin2θ(Type I in the XZ Plane) | |
| deff(II)=d31cosθ(Type II in the YZ Plane) | |
| deff(II)=d31cos2θ+d32sin2θ(Type II in the XZ Plane) | |
| NLO Sensitivity Does not Disappear | d31=1.05 ± 0.09 pm/V |
| d32=-0.98 ± 0.09 pm/V | |
| d33= 0.05 ± 0.006 pm/V | |
| Thermal-optical Coefficient(°C<,<λinμm) | dnx/dT=-9.3X10-6 |
| dny/dT=-13.6X10-6 | |
| dnz/dT=(-6.3-2.1λ)X10-6 | |
| Angle to Accept | 6.54mrad-cm(Φ,I型,1064 SHG)15.27mrad-cm(q,II型,1064 SHG) |
Linear Optical Properties
| Attribute | Numerical |
| Transparent Range | 169 – 2600 nm |
| Absorption Coefficient | <0.1%/cm @1064nm;<0.3%/cm @532nm |
| Refractive Index, | #colspan# |
| At 1.0642 mm | nx= 1.5656, ny= 1.5905, nz= 1.6055 |
| At 0.5321 mm | nx = 1.5785, ny = 1.6065, nz = 1.6212 |
| At 0.2660 mm | nx = 1.5973, ny = 1.6286, nz = 1.6444 |
| Sellmeier Equation(λ in μm) | nx2=2.454140+0.011249/(λ2-0.011350)-0.014591λ2-6.60×10-5λ4 |
| ny2=2.539070+0.012711/(λ2-0.012523)-0.018540λ2+2.0×10-4λ4 | |
| nz2=2.586179+0.013099/(λ2>-0.011893)-0.017968λ2-2.26×10-4λ4 |
Phase Matching Angle Experimental Value (T=293K)
| Interaction Wavelength[μm] | Φexp [deg] | θexp [deg] |
| XY Plane θ= 90° | ||
| SHG, o+o ⇒ e | ||
| 1.908⇒0.954 | 23.8 | |
| 1.5⇒0.75 | 7 | |
| 1.0796⇒0.5398 | 10.6/10.7 | |
| 1.0642⇒0.5321 | 11.3/11.4/11.6/11.8 | |
| 0.946⇒0.473 | 19.4/19.5 | |
| 0.930⇒0.465 | 21.3 | |
| 0.896⇒0.448 | 23.25 | |
| 0.88⇒0.44 | 24.53 | |
| 0.850⇒0.425 | 27 | |
| 0.84⇒0.42 | 27.92 | |
| 0.836⇒0.418 | 28.3 | |
| 0.80⇒0.40 | 31.7 | |
| 0.794⇒0.397 | 32.3 | |
| 0.786⇒0.393 | 33 | |
| 0.78⇒0.39 | 33.7 | |
| 0.7735⇒0.38675 | 34.4 | |
| 0.75⇒0.375 | 37.13/37 | |
| 0.746⇒0.373 | 37.5 | |
| 0.7094⇒0.3547 | 41.8/41.9/42/43.5 | |
| 0.63⇒0.315 | 55.6 | |
| 0.555⇒0.2775 | 86 | |
| 0.554⇒0.277 | 90 | |
| SFG, o+o ⇒ e | ||
| 1.3414+0.6707⇒0.44713 | 20 | |
| 1.0642+0.5321⇒0.35473 | 37/37.1/37.2 | |
| 1.053+0.5265⇒0.351 | 38.2 | |
| 1.0642+0.35473⇒0.26605 | 60.7/61 | |
| 0.86+0.43⇒0.2867 | 61 | |
| 1.3188+0.26605⇒0.22139 | 70.2 | |
| 0.21284+2.35524⇒0.1952 | 50.3 | |
| 0.21284+1.90007⇒0.1914 | 63.8 | |
| 0.21284+1.58910⇒0.18774 | 88 | |
| YZ Plane, Φ=90◦ | ||
| SHG, o+e ⇒ o | ||
| 1.908⇒0.954 | 46.2 | |
| 1.5⇒0.75 | 14.7 | |
| 1.0796⇒0.5398 | 19.2 | |
| 1.0642⇒0.5321 | 19.9/20.5/20.6/21.0 | |
| SFG, o+e⇒ o | ||
| 1.0641+0.53205⇒0.3547 | 42/42.7 | |
| 1.0642+0.5321⇒0.35473 | 42.2/42.5/43.2 | |
| XZ Plane, Φ=0◦, θ<VZ | ||
| SHG, e+o ⇒ e | ||
| 1.3414⇒0.6707 | 3.6/4.2/5.0 | |
| 1.3188⇒0.6594 | 5.2 | |
| 1.3⇒0.65 | 5.4 | |
| XZ Plane, Φ=0◦, θ>VZ | ||
| SHG, e+e ⇒ o | ||
| 1.3414⇒0.6707 | 86.1/86.3/86.6 | |
| 1.3188⇒0.6594 | 86 | |
| 1.3⇒0.65 | 86.1 | |
| 1.24⇒0.62 | 86 | |
Experimental Values of Non-critical Phase Matching (NCPM) Temperature
| Interaction Wavelength[μm] | T[℃] |
| Along the X-Axis SHG, typeⅠ | |
| 1.547⇒0.7735 | 117 |
| 1.46⇒0.73 | 50 |
| 1.252⇒0.626 | 3.5 |
| 1.25⇒0.625 | -2.9 |
| 1.215⇒0.6075 | 21 |
| 1.211⇒0.6055 | 20 |
| 1.206⇒0.603 | 24 |
| 1.2⇒0.6 | 24.3 |
| 1.15⇒0.575 | 61.1 |
| 1.135⇒0.5675 | 77.4 |
| 1.11⇒0.555 | 108.2 |
| 1.0796⇒0.5398 | 112 |
| 1.0642⇒0.5321 | 148/148.5/149/149.5/151 |
| 1.047⇒0.523 | 166.5/167/172/175/176.5/180 |
| 1.025⇒0.5125 | 190.3 |
| SFG, typeⅠ | |
| 1.908+1.0642⇒0.6832 | 81 |
| 1.444+1.08⇒0.6179 | 23 |
| 1.135+1.0642⇒0.5491 | 112 |
| 1.547+0.7735⇒0.5157 | 141 |
| DFG, typeⅠ | |
| 0.532-0.8⇒1.588 | 135 |
| Along the Z Axis SHG, type II | |
| 1.342⇒0.671 | 35 |
| 1.3⇒0.65 | 46 |
Spectrum
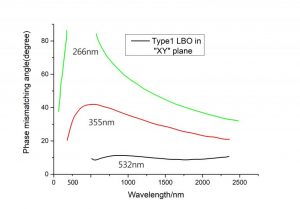 | 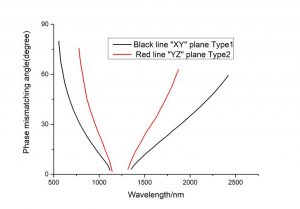 |
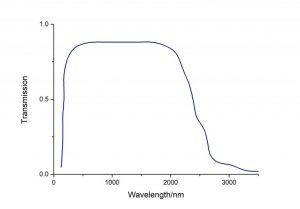 |
References
| [1] Zubrinov I I , Sapozhnikov V K , Pylneva N A , et al. Elastic and elastooptic properties of LiB 3O 5[J]. Ceramics International, 2004, 30(7):1675-1677. |
| [2] Reinvestigation on the phase transition of a LiB3O5 crystal near its melting point[J]. Journal of Crystal Growth, 2016, 435:1-5. |
| [3] A Y J , A C L J , A L W , et al. The optical properties of planar waveguides in LiB3O5 crystals formed by Cu+ implantation – ScienceDirect[J]. Applied Surface Science, 2006, 253( 5):2674-2677. |
| [4] Yang L , Yue Y , Mao Q , et al. Growth and nonlinear optical properties of Zn-doped LiB3O5 crystals[J]. Optical Materials, 2015, 43:6-9. |
| [5] Kananen B E , Mcclory J W , Giles N C , et al. Copper-doped lithium triborate (LiB3O5) crystals: A photoluminescence, thermoluminescence, and electron paramagnetic resonance study[J]. Journal of Luminescence, 2017:S0022231317309109. |
| [6] Shepelev Y F , Bubnova R S , Filatov S K , et al. LiB3O5 crystal structure at 20, 227 and 377°C[J]. Journal of Solid State Chemistry, 2005, 178(10):2987-2997. |
| [7] Nikolov I , Perlov D , Livneh S , et al. Growth and morphology of large LiB 3O 5 single crystals[J]. Journal of Crystal Growth, 2011, 331(1):1-3. |
| [8] Surovtsev N V , Malinovsky V K , Solntsev V P , et al. Peculiarities of LiB3O5 crystallization from melts studied by Raman spectroscopy[J]. Journal of Crystal Growth, 2008, 310(15):3540-3544. |
| [9] Ogorodnikov I N , Kruzhalov A V , Porotnikov A V , et al. Dynamics of electronic excitations and localized states in LiB3O5[J]. Journal of Luminescence, 1998, 76-77(2):464-466. |
| [10] I. N , Ogorodnikov, and, et al. Sub-nanosecond time-resolved spectroscopy of LiB3O5 under synchrotron radiation[J]. Journal of Luminescence, 1997. |
| [11] Depci T , Oezbayoglu G , Yilmaz A , et al. The thermoluminescent properties of lithium triborate (LiB3O5) activated by aluminium[J]. Nuclear Inst & Methods in Physics Research B, 2008, 266(5):755-762. |
| [12] Neumair S C , Vanicek S , Kaindl R , et al. High-pressure synthesis and crystal structure of the lithium borate HP-LiB3O5[J]. Journal of Solid State Chemistry, 2011, 42(52):no-no. |
| [13] Kannan C , Kimura H , Miyazaki A , et al. Nucleation, growth and characterization of LiBO single crystals[J]. Journal of Crystal Growth, 2005, 275(1–2):e769-e774. |
| [14] Lim A R , Yoon C S . Structural nature of 7Li and 11B sites in the nonlinear optical material LiB3O5 using static NMR and MAS NMR[J]. Materials Chemistry & Physics, 2014, 147(3):644-648. |
| [15] Li H Q , Zhang H B , Bao Z , et al. High-power nanosecond optical parametric oscillator based on a long LiB3O5 crystal[J]. Optics Communications, 2004, 232(1-6):411-415. |
| [16] Kannan C , Ganesamoorthy S , Rajesh D , et al. Anisotropic properties of self-flux grown LiB 3O 5 single crystals[J]. Solid State Communications, 2005, 136(4):215-219. |
| [17] Ogorodnikov I N , Isaenko L I , Kruzhalov A V , et al. Thermally stimulated luminescence and lattice defects in crystals of alkali metal borate LiB3O5 (LBO)[J]. Radiation Measurements, 2001, 33(5):577-581. |
| [18] Sabharwal S C , Tiwari B , Sangeeta. Investigations on the growth of LiB 3 O 5 crystal by top-seeded solution growth technique[J]. Journal of Crystal Growth, 2004, 263(1/4):327-331. |
| [19] Sabharwal S C , Tiwari B , Sangeeta. Effect of highest temperature invoked on the crystallization of LiB 3O 5 from boron-rich solution[J]. Journal of Crystal Growth, 2003, 249(3):502-506. |
| [20] Almeida A , Thomazini D , Vasconcelos I F , et al. Structural studies of lithium triborate (LBO–LiB3O5) in borophosphate glass-ceramics[J]. International Journal of Inorganic Materials, 2001, 3(7):829-838. |
Standard Products
| Product | Size (W*L*T, mm) | Type | Coating |
| LBO | 2*2*10 | θ=90°, φ=11.4° | AR@1064&532nm |
| LBO | 2*2*10 | θ=90°, φ=11.3° | AR@1064&532nm |
| LBO | 3*3*5 | θ=90°, φ=36.1° | AR@1064&532nm&355nm |
| LBO | 3*3*8 | θ=90°, φ=9.6°, 65C | AR@1064&532nm |
| LBO | 3*3*8 | θ=90°, φ=11° | AR@1064&532nm |
| LBO | 3*3*8 | LBO 3*3*8 θ=90°, φ=36.1° | AR@1064&532nm&355nm |
| LBO | 3*3*10 | θ=90°, φ=36.1° | AR@1064&532nm&355nm |
| LBO | 3*3*15 | θ=90°, φ=0° | AR@1064&532nm |
| LBO | 3*3*15 | θ=42.8°, φ=90°, single brewster wedge 57.4° | S1-AR@1064&532nm |
| LBO | 3*3*20 | θ=44.8°, φ=90°, 65C, single brewster wedge 57.42° | S1-AR@1064&532nm |
| LBO | 3*3*20 | θ=44°, φ=90°, single brewster wedge 57.4° | S1-AR@1064&532nm |
| LBO | 4*4*13 | θ=44°, φ=90° | AR@1064&532&355nm |
| LBO | 4*4*15 | θ=43.3°, φ=90° | AR@1064&532&355nm |
| LBO | 4*4*16 | θ=44°, φ=90°, single brewster wedge58° | S1-AR@1064&532nm |
| LBO | 5*5*15 | θ=90°, φ=9.9° | AR@1064&532nm |
| LBO | 5*5*15 | θ=90°, φ=8.6°, 60C | AR@1053nm&527nm |
| LBO | 5*5*17 | θ=90°, φ=8.6°, 60C | AR@1053nm&527nm |
| LBO | 5*5*19 | θ=90°, φ=10.7° | AR@1053&527nm |
| LBO | 5*5*19 | θ=90°, φ=8.6° AR@ | AR@1053&527nm |
| LBO | 6*6*1 | θ=54.48°, φ=0° | AR@640nm |
| LBO | 10*10*3 | θ=90°, φ=11.5° | AR@1064&532nm |
| LBO | 10*10*15 | θ=90°, φ=11.3°, 47C | AR@1064&532nm |
| LBO | 25*25*20 | θ=90°, φ=11.4° | AR@1064nm&532nm |
| LBO | 25*25*20 | θ=90°, φ=12° | AR@1064nm&532nm |
If you are intereted in LBO (Nonlinear Crystal), please click the button below to enquire or ask for a samplel.
Related article(s) with LBO (Nonlinear Crystal):
Related case study with LBO (Nonlinear Crystal):
Related solution(s) with LBO (Nonlinear Crystal):
Related video(s) with LBO (Nonlinear Crystal):
There is no related video(s), please visit videos page to find out more.